The event gathered over 100 participants from different regions of Kazakhstan, as well as 25 speakers, offline and online. The conference brought together leading pharmaceutical industry experts, representatives of regulatory authorities, distributors, and manufacturers. The discussions covered the macroeconomic outlook, regulatory changes, budgeting and planning strategies, andes, budgeting and planning strategies, as well as the key challenges facing the pharmaceutical market in 2026. Proxima Research and Medical Data Management organized the event.
Speakers from the pharmaceutical industry, government bodies, distribution and pharmacy organizations, and industry associations presented practical case studies, analytical forecasts, and modern approaches to budgeting.
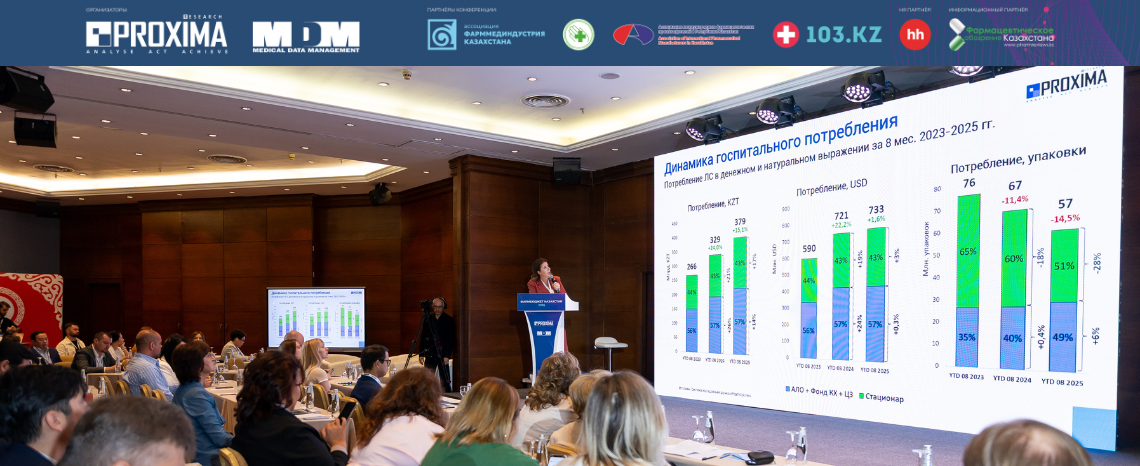
Each session opened with an analytical review of the pharmaceutical market for the first 8 months of 2025, presented by Ekaterina Markova, Director of Proxima Research Kazakhstan.
Key macroeconomic indicators, including inflation trends and exchange rates, as well as the impact of regulatory initiatives on the market, were discussed. Participants explored prospects for accelerated drug registration, ways to reduce the time it takes to bring innovative medicines to patients, and the role of local production in improving therapy accessibility.
The first session concluded with a discussion involving representatives of the Ministry of Health of the Republic of Kazakhstan, SK-Pharmacy, the National Center for Expertise of Medicines and Medical Devices, the Republican Center for Electronic Healthcare, the National Scientific Center for Health Development named after S. Kairbekova, as well as representatives of leading pharmaceutical companies. The debate focused on “Pharmabudget 2026: Hospital and Retail, Between Growth and Savings — Where to Place the Bet?”. The participants examined the balance between hospital and retail segments’ needs, cost optimization mechanisms, and potential market development scenarios under limited resources. The discussion also discussed the practical aspects of applying new regulatory mechanisms, possible risks and benefits of accelerated registration, and prospects for local and international manufacturers in the changing economic environment.
The second session focused on modern analytics tools and digital solutions for the pharmaceutical industry. Experts provided an in-depth look at Market Audit and PromoTest & RxTest systems, which allow monitoring of market dynamics and promotional effectiveness, and discussed approaches to pharmaceutical market forecasting for 2026. Another key area was the automation of pharmacy processes with Proxima Apteka and Pharmacy BI solutions, which enhance transparency and business management.
Particular attention was given to the use of artificial intelligence in planning, which opens new perspectives for data analysis and faster decision-making. Practical case studies demonstrated how the implementation of digital tools helps companies optimize marketing costs, reduce operating expenses, and build more accurate customer engagement strategies. Participants noted that such solutions are becoming a key factor not only in saving resources but also in ensuring sustainable development of pharmaceutical companies in an increasingly competitive environment.
The key event of the third session was the panel discussion “Voice of the Market: Distribution and Pharmacies – New Rules of the Game and Budgeting Risks”, which brought together representatives of distribution companies, pharmacy chains, manufacturers, industry associations, and organizations involved in the digitalization of the pharmaceutical sector. The discussion focused on the implementation of the drug labeling and traceability system, new regulatory requirements, and their impact on business processes. Experts noted that these changes, on the one hand, create opportunities to increase transparency across the supply chain and protect patients from counterfeit medicines, but on the other hand, they generate additional costs for market participants and require a revision of traditional budgeting models.
The session program included several substantial presentations: “Integration of Systems and Decentralization of ALO: Strategy and Implementation” — an overview of promising directions for the development of digital healthcare and coordination of processes at the state level. “The Path of a Medicine from Production to the Pharmacy” — an analysis of the practical aspects of logistics and the key links in the supply chain that determine the speed and availability of medicines for the end consumer. “Drug Labeling and Traceability” — a detailed examination of the stages of implementing the national traceability system and its impact on market participants. “Automation and Digital Tools” — a presentation of solutions that help optimize interactions between manufacturers, distributors, and pharmacies, reduce operating costs, and ensure process transparency.
Particular attention was given to the integration of digital solutions that enable end-to-end visibility of product movement from manufacturer to pharmacy shelf, minimize the risks of parallel imports, and optimize inventory management. Participants highlighted that the new conditions require a restructuring of relationships between key market players and the search for mechanisms that allow balancing rising costs with maintaining patient access to therapy.
The discussion also featured practical case studies of the implementation of digital tools in logistics and retail, examples of business process optimization, and new formats of collaboration that contribute to the creation of sustainable interaction models.
Experts emphasized that distribution and pharmacies are currently at the forefront of transformation: intensifying competition, strict regulations, and the need for accelerated digitalization simultaneously create risks for business sustainability while opening pathways to innovation. The discussion concluded that the future of Kazakhstan’s pharmaceutical market will largely depend on how effectively distributors and pharmacy chains can adapt to the “new rules of the game” and find a balance between financial sustainability and ensuring access to medicines for the population.
Experts analyzed changes in the pharmaceutical labor market, new communication channels with patients, and the development of online platforms. The discussions also covered the prospects of remote training for specialists, the introduction of digital tools to improve process efficiency, and approaches to managing advertising budgets with a focus on KPI control.
Equally dynamic were debates on the future of distribution and pharmacies, where participants discussed the balance between growth and cost-saving. Experts emphasized that 2026 will be a year of major challenges: rising competition, strict regulations, and the need for accelerated digitalization of business processes. At the same time, strategies were presented that demonstrate real opportunities for sustainable growth and adaptation of companies to the new conditions.
The “PHARMABUDGET KAZAKHSTAN 2026” conference became a significant platform for dialogue between government representatives, businesses, and industry associations. The event brought together market participants to seek solutions that will help ensure the financial sustainability and development of the pharmaceutical sector under strict regulations.
Stay tuned on Proxima Research’s Telegram and Instagram to follow upcoming events.
By clicking the “Subscribe” button, you consent to the processing of personal data and the receipt of electronic messages about Proxima Research products and services, and you agree to our Terms of Use. Your data will be processed in accordance with our Privacy Policy. You can unsubscribe at any time.
or